Abstract
Introduction: In previous work, we have demonstrated that culturing leukemia cell lines in decellularized Wharton's jelly (WJ) matrix (DWJM), the gelatinous material in umbilical cord tissues, resulted in chemotherapy resistance. To reduce the variability in the biochemical composition between different parts of the WJ matrix and to optimize our 3-dimensional (3D) DWJM-based extracellular matrix (ECM) model for in vitro culture of primary AML cells, we fabricated DWJM-derived porous disks with uniform architecture and pore-size by homogenization followed by lyophilization of human DWJM. Herein, we examine whether DWJM disks support primary leukemia cells and result in chemotherapy resistance.
Methods: AML patient samples collected by leukapheresis were cultured in DWJM disks for one week. Non-adherent cells were first aspirated and adherent cells were separately isolated and assessed for viability, apoptosis, and colony forming unit (CFU). RNA sequencing was performed at the end of culture. Response to chemotherapy following treatment with doxorubicin was also assessed. For all studies, DWJM-adherent and non-adherent cells were compared to suspension culture controls. Metabolic pathway analyses were conducted using enzyme-linked immunosorbent assay (ELISA). One-tailed t-test was used for comparison between the groups.
Results: Consistent with our prior studies, adherent DWJM cells demonstrated less apoptosis (P=0.027) and greater CFU activity with larger and more dense colonies (1 vs 4/50,000 cells, P=0.0008). Co-culture of primary AML samples with DWJM reduced doxorubicin induced cell death (P=.047) preserving CFU activity (3.3 vs 0.3/300,000 cells, P=.047) compared to treatment of suspension culture cells. To understand the mechanisms by which co-culture with DWJM enhanced leukemic progenitor function and therapy resistance, we performed RNA-Seq analyses. RNA-Seq data analysis from day 7 demonstrated significant upregulation of FAM83A and MIR34A and downregulation of BPI, ZNF521, NHLH2, CD69, FKBP14, PBX1, TANC1, GRIN2b, MYO6, INHBA, SA1008, CXCL1, A1009, BLNK, MMP9, BHLHE41, and CD9 in the adherent population compared to the suspension population (adjusted P-value <0.05). Ingenuity Pathway Analysis (IPA) identified glutamate receptor signaling as the top impacted canonical pathway, suggesting differences in the predominant metabolic process between the two culture conditions. Additionally, IPA showed that FAM83A was the most upregulated molecule, while MT-TH and MT-TW were two of the most down-regulated molecules in the adherent cells. The DWJM culture condition was associated with a significant increase in lactate (P=0.020) and significant reduction in glucose (P=0.005) in culture supernatants compared to suspension controls.
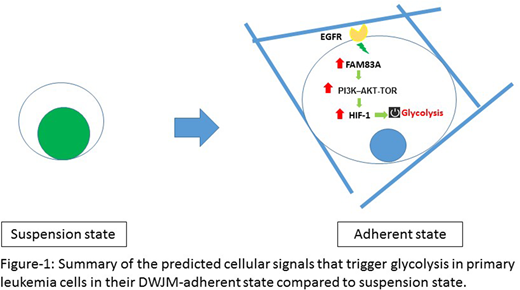
Conclusion: We demonstrate that DWJM disks support primary leukemia cell survival. DWJM-adherent cells demonstrate chemotherapy resistance in association with induction of glycolysis. Based on our RNA Seq data we hypothesize that leukemia cell adherence to DWJM upregulates FAM83A, which functions in the epidermal growth factor receptor (EGFR) signaling pathway. FAM83A is known to control PI3K-AKT-TOR signaling cascade. M-TOR, in turn is known to activate HIF-1α pathway, which regulates glycolysis (Figure-1). Additionally, down-regulation of mitochondria-related molecules (MT-TH and MT-TW) is consistent with a switch from oxidative phosphorylation to glycolysis in the adherent cells. Further work is ongoing to further understand the role of glycolysis in primary leukemia cell chemotherapy resistance.
Lipe:Celgene: Consultancy. Aljitawi:The University of Rochester Medical Center: Patents & Royalties: Pending patent related to decellularized Wharton's jelly matrix; Medpace: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal